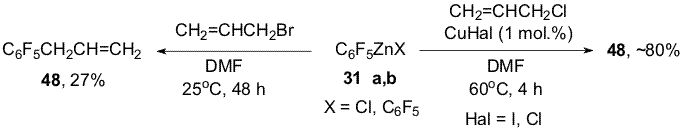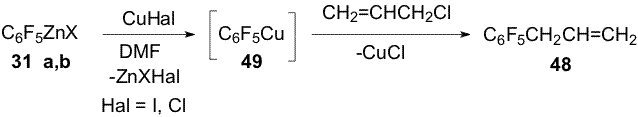Fluorine Notes, 2009, 66, 5-6
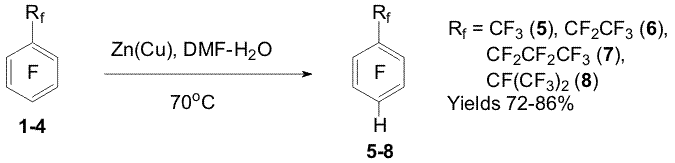
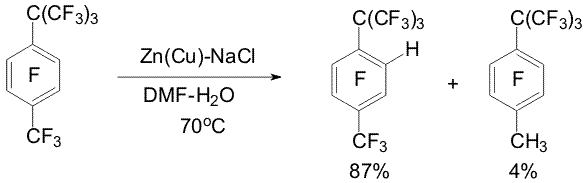
POLYFLUOROARENES AND Zn: HYDROGENOLYSIS REACTIONS, SYNTHESIS AND CHEMICAL PROPERTIES OF POLYFLUOROAROMATIC ORGANOZINC COMPOUNDSV.E.Platonov, A.S.Vinogradov, V.I.Krasnov Novosibirsk N.N. Vorozhtsov Institute of Organic Chemistry of SB RAS, Among chemical properties of polyfluorinated aromatic compounds the reactions with nucleophilic, electrophilic, radical reagents and carbenes have been studied in sufficient details. The processes initiated by electron transfer have not been studied adequately yet. The reactions of hydrodehalogenation of polyfluoroarenes under the action of metals and the reactions of formation of polyfluoroaryl metallo-organic compounds may be ascribed to these processes. We found a convenient reducing system Zn(Cu) in aqueous dimethylformamide (DMF) to study regularities of the reactions of polyfluoroarenes hydrodehalogenation as well as conditions for obtaining polyfluoroaryl organozinc compounds from polyfluoroarenes and zinc. Systematic investigation of influence of the chemical structure of polyfluoroarenes on the rate and regioselectivity of their hydrodehalogenation as well as formation of polyfluoroaryl organozinc compounds is important for the study of these processes regularities running with probable participation of anion radicals. From the synthetic point of view the investigation urgency lies in modification of perfluoroalkyl substituents, inert to the action of many reagents, by means of the hydrodefluorination reactions as well as in development of an approach to obtain partially fluorinated arenes by hydrogenolysis of C-F bonds of the aromatic ring of perfluoroarenes and hydrogenolysis of C-Cl bonds of polychloroarenes. Polyfluoroaryl organozinc compounds may be used to obtain various functional derivatives of polyfluoroarenes. 1.Perfluoroarenes hydrodefluorination and perfluorochloroarenes hydrodechlorination under the action of Zn(Cu) in aqueous DMF Perfluoroalkylbenzene series containing CF3 (1), CF2CF3 (2), CF2CF2CF3 (3), CF(CF3)2 (4) were found to exhibit high regioselectivity of hydrodefluorination under the action of Zn(Cu) in aqueous DMF [1-3]. The reaction takes place in the para position of the aromatic ring while the rest bonds, including C-F bonds, are in the benzyl position. Addition of electrolytes (NaCl, CaCl2 etc.) accelerates the hydrodefluorination (Scheme 1).
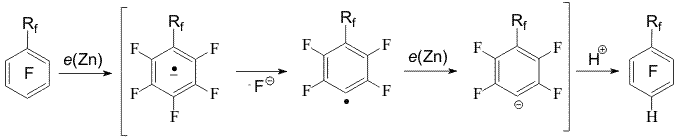
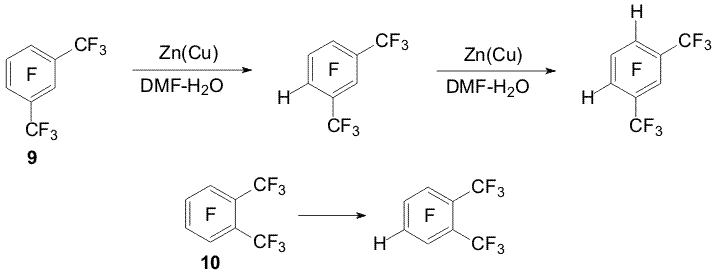
Scheme 1 It was proposed that the hydrodefluorination mechanisms included the formation of the anion-radical of polyfluoroarene and detachment of fluoride ion from it [2].The forming radical possesses high electron affinity [4] and is reduced to a carbanion resulting in a hydro derivative in a protonic media [2,5] (Scheme 2). The presence of the perfluoroalkyl group of a great volume (perfluoro-tert-butyl group) may favour replacement of the ortho fluorine atom by hydrogen. At that regioselectivity of perfluoro-tert-butylbenzene hydrodefluorination depends on the ratio of DMF and water. The prevailing formation of the ortho-hydro derivative (64%) was observed at 46 % (mole) of water, whereas at 83 % (mole) of water prevailed the formation of the para-hydro derivative (99%) [3]. In perfluorinated m- and o-xylenes 9, 10 under the action of Zn(Cu) the fluorine atoms of the aromatic ring in the para-position towards to the CF3-group are replaced by hydrogen [6] (Scheme 3).
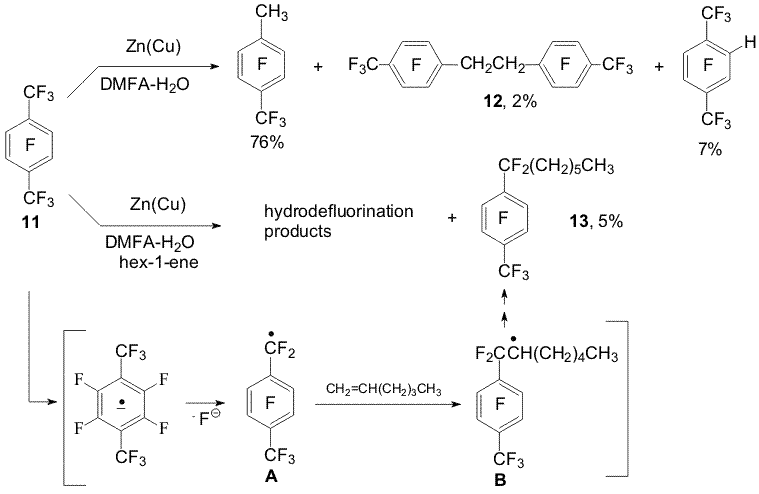
Scheme 3 The hydrogenolysis mechanism of the aryl C-F bonds in the present perfluoroxylenes is similar to the mentioned above for perfluoroalkylbenzenes. The main direction of the reaction of perfluoro-p-xylene (11) with Zn(Cu)-DMF-H2O is conversion of the trifluoromethyl group into methyl one as well as formation of 1,2-bis(4-trifluoromethyl-2,3,5-tetrafluorophenyl)ethane (12); the hydrogenolysis of the C-F bond of the aromatic ring takes place to a lesser degree in comparison with the hydrogenolysis of the C-F bond of the CF3-group [6]. Compound 12 is not formed in the presence of hexane-1. Whereas compound 13 has been found probably forming as a result of addition of benzyl type radical (A) to the double bond of hexene-1 followed by reduction of new radical (B) into the corresponding carbanion (Scheme 4). Scheme 4 The estimation of prospective fragmentation direction of anion radicals of perfluoroxylenes
based on consideration of the structure and energies of molecular orbitals
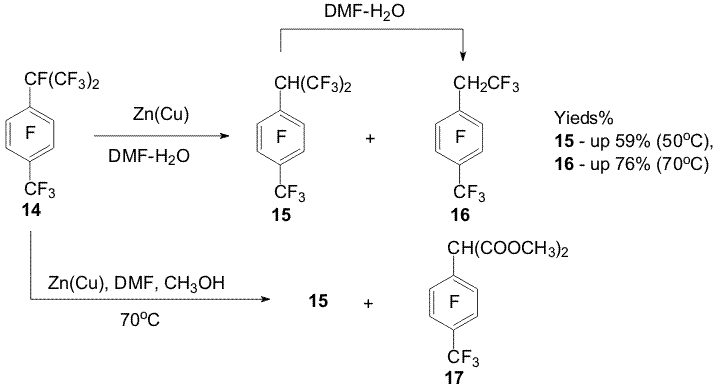
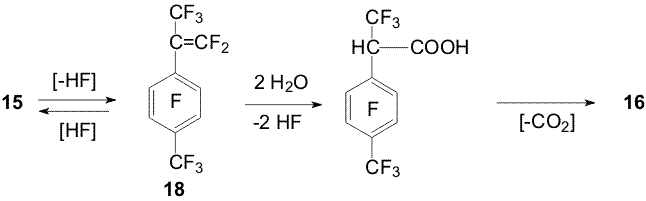
of the ground The hydrofluorination of perfluoro-4-tert-butyltoluene under the action of Zn(Cu) takes place predominantly in the ortho position towards the perfluoro-tert-butyl group, the trifluoromethyl group is less affected by the process [3,7,8] (Scheme 5). Scheme 5 Only the heptafluoroisopropyl group in perfluoro-p-cymene (14) undergoes transformation [6]. It was managed to obtain hexafluoroisopropyl derivative 15 along with trifluoroethyl derivative 16 at 50oC under the action of Zn(Cu) in DMF-H2O. The latter is obtained in a good yield at 70-90oC. Compound 16 is formed from derivative 15 at heating in aqueous DMF. Trifluoroethyl derivative 16 was not found under the action of Zn(Cu) on cymene 14 in a DMF-methanol mixture and first forming cymene 15 yields a derivative of malonic acid 17 [5,7,8] (Scheme 6). Scheme 6 These data along with transformation of perfluoro-α,4-dimethylstyrene (18) in aqueous DMF ( including in the presence of Zn(Cu)) into products 15 and 16 makes possible to suggest a scheme of formation of the trifluoroethyl group via intermediate styrene 18 (Scheme 7).
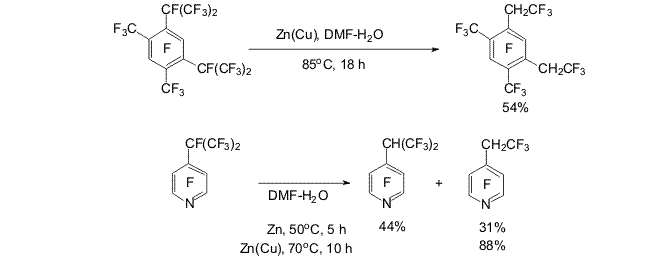
Scheme 7 Transformations with formation of CH(CF3)2 and(or) CH2CF3 derivatives of perfluoroarenes occurs in the reactions of compounds containing CF(CF3)2 group in the para position towards trifluoromethyl, nitrile groups or heteroatom [8-10] (Scheme 8).
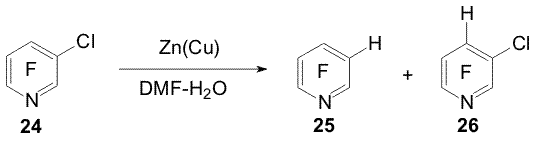
Scheme 8 2,3,5,6-Tetrafluoropyridine was obtained from pentafluoropyridine (19)
under the action of Zn(Cu)-DMF-H2O at 70oC. Octafluoronaphthalene
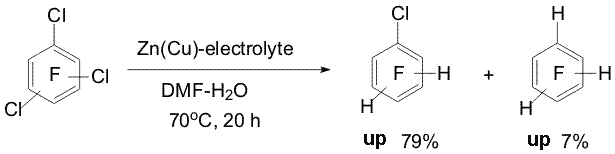
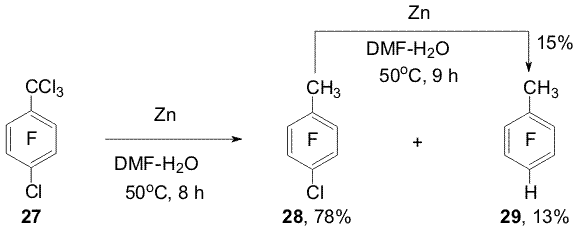
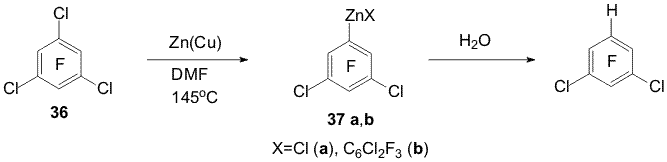
(20) under similar conditions yields a mixture of α- and Reactions of some perfluoroarenes under the action of Zn(Cu) may be carried out in water in the absence of organic solvent. In this case the direction of hydrodefluorination of compounds 1, 9, 11, 14, 19-21 is the same as in aqueous DMF. Using electrolytes is necessary to carry the reactions with the mentioned substrates ( except pyridine 19) [5,11]. The reactions of selective hydrodechlorination of polyfluorochloroarenes under the action of Zn or Zn(Cu) are of a synthetic interest for obtaining partially fluorinated arenes taken into account that polyfluorochloroarenes are readily obtained by replacement of chlorine by fluorine in perchloroarenes. Easiness of hydrodehalogenation in series of C6F5-Hal compounds varies as C6F5-Br > C6F5-Cl > C6F5-F. The conversion of chloropentafluorobenzene (22) into pentafluorobenzene (23) already does not require using Zn(Cu) and can be executed by means of Zn. Partial hydrodechlorination of a mixture of o-, m-, p-dichlorotetrafluorobenzenes to form chlorotetrafluorobenzenes occurs when dichlorotetrafluorobenzenes are treated with Zn in aqueous DMF at 20oC or in boiling with Zn in aqueous NaCl solution. Complete hydrodechlorination into tetrafluorobenzenes takes place under the action of Zn(Cu) in DMF-H2O at 70oC, in this case addition of electrolytes (NaCl, Na2SO4, NH4Cl) promotes the reaction [7, 12]. The action of Zn (Cu) on a mixture of 1,2,3-, 1,2,4- and 1,3,5-trifluorotrichlorobenzenes in aqueous DMF at 70oC both in the absence and presence of salts brings predominantly to obtaining chlorotrifluorobenzenes [7,12] (Scheme 9). Isomeric mixtures of dichlorotetrafluoro- and trifluorotrichlorobenzenes were obtained in the reaction of hexachlorobenzene with KF [13]. Scheme 9 Scheme 10 Benzyl C-Cl bonds in 1-trichloromethyl-2,3,5,6-tetrafluoro-4-chlorobenzene (27) more easy undergo hydrodechlorination under the action of Zn-DMF-H2O than the C-Cl bond of the aromatic ring [12] (Scheme 11).
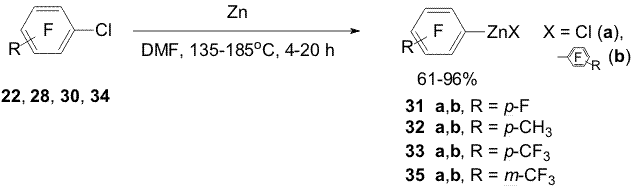
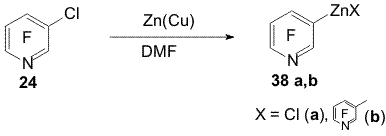
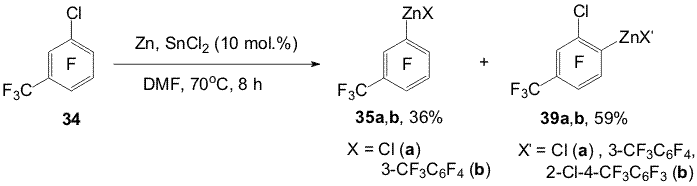
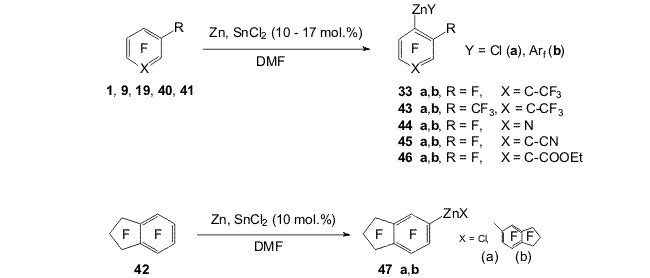
Scheme 11 2. Obtaining polyfluoroaromatic organozinc compounds From compounds 22, 4-chloro-2,3,5,6,-tetrafluorotoluene (28), 4-chloroheptafluorotoluene (30) and Zn in DMF were obtained organozinc compounds with participation of the C-Cl bond (31a,b-33a,b) [44] (Scheme 12). Scheme 12 Compounds 22 and 30 practically entirely are converted into
organozinc reagents at 135oC (4 h) in contrast to chlorofluorotoluene
28 that does not react with Zn under similar conditions. The formation
of organozinc reagents 32a,b from compound 28 requires a prolong
heating with zinc powder at elevated temperatures and is followed by
the formation of a noticeable amount of 2,3,5,6-tetrafluorotoluene (29).
Organozinc compounds 35a,b are formed from 3-chloroheptafluorotoluene
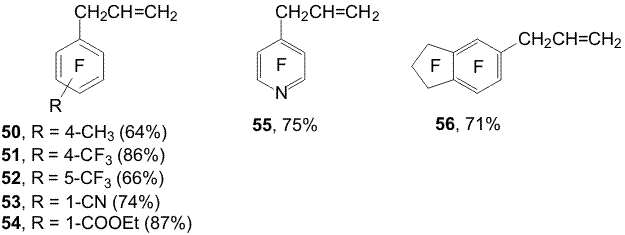
(34) and Zn ( Scheme 12). Scheme 13 Organozinc compounds with participation of the C-Cl bond (38a,b) are obtained from chlorofluoropyridine 24 in the reaction with Zn(Cu) in DMF [1,12](Scheme 14). Scheme 14 An addition of 10 mole % SnCl2 to Zn in the reaction with chlorofluoroarene 34 changes the process direction and organozinc compounds 39a,b are obtained with participation of the C-F bond in the para-position towards the CF3-group as main products along with smaller quantity of organozinc compounds 35a,b forming by insertion of Zn by the C-Cl bond [15](Scheme 15). Scheme 15 When perfluoroarenes 1,9,19, pentafluorobenzonitrile (40), ethyl pentafluorobenzoate(41) and decafluoroindane (42) are used in the reactions with Zn to obtain organozinc compounds 33(a,b), 43 (a,b)-47(a,b) there is used SnCl2 as a catalyst [14,16] (Scheme 16). Scheme 16 3. Reactions of polyfluoroaryl organozinc compounds with allylhalides The reaction of organozinc compounds 31a,b with allyl bromide takes place at room temperature to afford allylpentafluorobenzene (48)[14]. This process may be considered as nucleophilic replacement of the bromine atom in allylbromide with the pentafluorophenyl group. In contrast to allylbromide the reaction with allylchloride almost does not occur. It has been shown that salts of univalent copper (CuCl, CuI) accelerate the process with allylchloride and compound 48 is obtained in a ~80% yield (Scheme 17). Scheme 17 The catalytic role of the salts is likely comes to their interaction with C6F5ZnX via intermediate formation of an organocopper compound 49. The latter reacts with allylchloride and apparently yields derivative 48 (Scheme 18). Scheme 18 Copper chloride was also used as a catalyst for obtaining 1-allyl-4-methyl-2,3,5,6-tetrafluorobenzene (50), 1-allyl-4-trifluoromethyl-2,3,5,6-tetrafluorobenzene (51), 1-allyl-5-trifluoromethyl-2,3,4,6-tetrafluorobenzene (52), 4-allyl-2,3,5,6-tetrafluorobenzonitrile (53), 4-allyl-2,3,5,6-tetrafluoroethylbenzoate (54), 4-allyl-2,3,5,6-tetrafluoropyridine (55) and 5-allylnonafluoroindane (56) in good yields from allylchloride and organozinc compounds 32 (a,b), 33(a,b), 35(a,b), 44(a,b) - 47(a,b). 4. Reactions of polyfluoroaryl organozinc compounds with acylchlorides The interaction of compounds 31a,b with chloroanhydrides of acetic
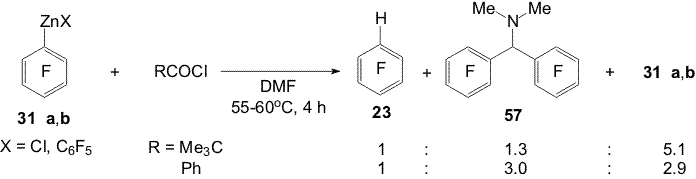
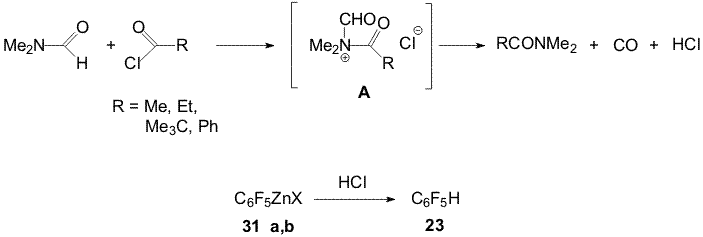
and propionic acids result in hydroderivative 23[17]. Scheme 19 Compound 23 is possibly obtained at the expense of the reamidation reaction with intermediate formation of ammonium cations (A) and their conversion into amides and unstable formyl chloride dissociating to CO and HCl. The latter reacts with organozinc reagent to afford 23 (Scheme 20). Scheme 20 Another direction of the reaction of pivaloyl- and banzoyl chlorides with DMF is apparently the interaction of electrophilic centers of these chloroanhydrides with the oxygen atom of DMF with intermediate formation of Vilsmeier reagents (B or C). Amine 57 is probably obtained with participation of these reagents that is shown in Scheme 21:
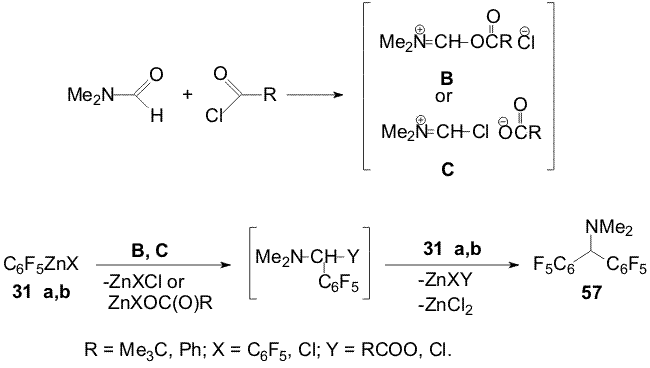
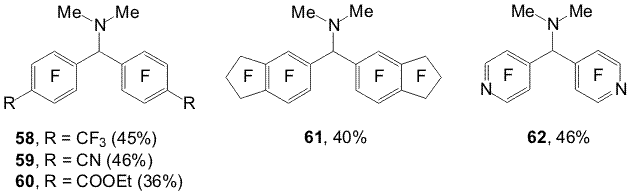
Scheme 21 Polyfluoroaromatic organozinc compounds 33a,b , 44 (a,b) - 47(a,b) react with the benzoylchloride excess in DMF also with formation of appropriate N,N-dimethyl-bis(polyfluoroaryl)methaneamines 58-62 [17,18]. Carrying out the reactions of compounds 31a,b with acylchlorides in
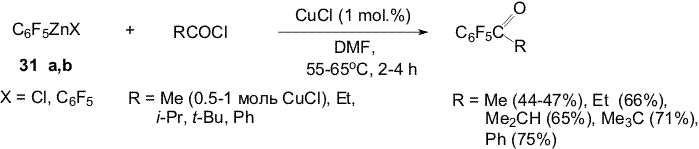
the presence mainly of CuCl in small quantities already changes the main
conversion direction and polyfluoroaromatic ketones are formed instead
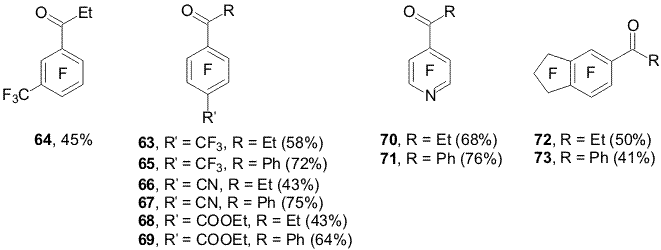
of amine 57. The reaction of propionylchloride with organozinc compounds 33a,b and 35a,b in the presence of CuCl ( 1 mole %) results in 4-propionyl- (63) and 3-propionylheptafluorotoluene (64) accordingly [19]. 4-Benzoylheptafluorotoluene (65) was synthesized from reagent 33a,b and benzoylchloride. The reaction of organozinc compounds 45a,b with propionyl- and benzoyl
chlorides in the presence of CuCl afford 4-propionyl- (66) and
benzoyl-2,3,5,6-tetrafluorobenzonitrile (67) accordingly, and
ethyl 4-propionyl- (68) and ethyl 4-benzoyl-2,3,5,6-tetrafluorobenzoate
(69) are obtained from organozinc compounds 46a,b. In a similar way from compounds 47a,b with propionyl- and benzoylchlorides there were produced 5-propionyl-(72) and 5-benzoyl-1,1,2,2,3,3,4,6,7-nonafluoroindane (73)[19]. The change in the main direction of the reaction of polyfluoroaryl organozinc compounds with acylchlorides in DMF in the presence of CuCl can be explained by means of the remetallation process and intermediate formation of polyfluoroaryl organocopper compounds participating in obtaining polyfluoroaromatic ketones (Scheme 23). Scheme 23 5. Reactions of polyfluoroaryl organozinc compounds with perfluoroarenes Polyfluoroaryl organozinc compounds at heating with polyfluoroarenes in DMF form symmetrical and asymmetrical polyfluorodiaryls [20].The orientation of the entering polyfluoroaryl group corresponds to that observed in the nucleophilic reactions of these perfluoroarenes [21] (Scheme 24). Scheme 24 Organozinc compounds 31a,b and pentafluoronitrobenzene give 4-(pentafluorophenyl)-2,3,5,6-tetrafluoronitrobenzene with a touch of 2-(pentafluorophenyl)-3,4,5,6-tetrafluoronitrobenzene. Thus, the hydrodehalogenation reaction of perfluoro- and polyfluorochloroarenes
under the action of Zn(Cu) in aqueous DMF makes possible to obtain partially
fluorinated aromatic compounds. By means of the reactions of polyfluoroaryl
organozinc compounds with electrophilic reagents the following compounds
can be produced: allylpolyfluoroarenes, N,N-dimethyl-bis(polyfluoroaryl)methaneamines,
polyfluoroaromatic ketones as well as symmetrical and asymmetrical polyfluorodiaryls. References 1. Krasnov V.I., Platonov V.E. Reactions of polyfluoroarenes with zinc: hydrogenolysis and synthesis of polyfluoroaromatic organozinc compounds // J. Fluor.Chem. 1992, 58, 246. 2. Краснов В.И., Платонов В.Е. Гидрогенолиз Саром.-F связей в поли- фторароматических соединениях под действием Zn(Cu) // ЖОрХ. 1993, 29, 1078. 3. Краснов В.И., Платонов В.Е. Восстановительные превращения фторорганических соединений. III. Гидродефторирование перфторалкилбензолов действием Zn(Cu). Необычное поведение соединений, содержащих перфтор-трет-бутильную группу // ЖОрХ. 2001, 37, 552. 4. Teherani T., Bard A.J. Electrochemistry in liquid ammonia. VII. Halonitrobenzenes // Acta Chem.Scand. 1983, 37B, 413. 5. Краснов В.И. Изучение превращений полифторароматических соединений под действием цинка или цинка в присутствии меди в водном диметилформамиде. Дис. канд. хим. наук. Новосибирск. 1999. 6. Krasnov V.I., Platonov V.E., Beregovaya I.V., Shchegoleva L.N. Transformations of perfluoroxylenes and perfluoro-p-cymene under the action of Zn(Cu)-DMF-H2O // Tetrahedron.1997, 53, 1797. 7. Платонов В.Е., Краснов В.И. Синтетические аспекты гидрогенолиза фторорганических соединений под действием Zn(Cu) // ЖОрХ. 1994, 30, 1271. 8. Platonov V.E., Krasnov V.I. Electron transfer in the polyfluoroarene chemistry: reactions of hydrogenolysis of perfluoroalkylbenzenes under action of Zn(Cu) in aqueous dimethylformamide // The 14th International Symposium on Fluorine Chemistry, Yokohama (Japan). 1994. Scientific Program and Abstracts. P. 133. 9. Krasnov V.I., Platonov V.E. Hydrogenolysis of polyfluoroisopropylaromatic compounds // J. Fluor.Chem. 1991, 54, 139. 10. Краснов В.И., Платонов В.Е. Гидрогенолиз гептафтор- и гексафторизопропильных групп в полифторароматических соединениях // Изв. АН СССР.Сер.хим. 1991, 2158. 11. Krasnov V.I., Platonov V.E. Hydrogenolysis of C-F, C-Cl bonds in polyfluoroarenes under the action of Zn(Cu) in aqueous medium and in the presence of electrolytes. // 15th International Symposium on Fluorine Chemistry, Vancouver (Canada).1997. Program and Abstracts. F-Ru IL-29. 12. Краснов В.И., Платонов В.Е. Восстановительные превращения фторорганических соединений. II. Гидродехлорирование полифторхлораренов цинком // ЖОрХ. 2000, 36, 1524. 13. Ворожцов-мл. Н.Н., Платонов В.Е., Якобсон Г.Г. Получение гексафторбензола из гексахлорбензола // Изв. АН СССР. Сер. хим. 1963, 1524. 14. Виноградов А.С., Краснов В.И., Платонов В.Е. Цинкорганические реагенты из полифтораренов и цинка: получение и реакции с аллилгалогенидами. Синтез аллилполифтораренов //ЖОрХ. 2008, 44, 101. 15. Krasnov V.I., Vinogradov A.S., Platonov V.E. Preparation of organozinc compounds from 3-chloroheptafluorotoluene and zinc: the participation of C-F bonds // Mendeleev Commun. 2006, 168. 16. Miller A.O., Krasnov V.I., Peters D., Platonov V.E., Miethchen R. Perfluorozinc aromatics by direct insertion of zinc into C-F or C-Cl bonds // Tetrahedron Lett. 2000, 41, 3817. 17. Vinogradov A.S., Krasnov V.I., Platonov V.E. Reactions of polyfluoroaromatic organozinc compounds with acyl chlorides and DMF // Coll. Czech. Chem. Commun. 2008, 73, 1623. 18. Vinogradov A.S., Krasnov V.I., Platonov V.E. Unusual interaction of perfluoroaromatic organozinc compounds with benzoyl chloride and DMF. // Mendeleev Commun.2008, 227. 19. Виноградов А.С., Краснов В.И., Платонов В.Е. Реакции полифтор- арилцинкорганических соединений с аллилгалогенидами и хлорангидридами карбоновых кислот // 7-я Всероссийская конференция ″Химия Фтора″, Москва (Россия). 2006. Сборник тезисов. Р-57. 20. Виноградов А.С., Краснов В.И., Платонов В.Е. Синтез перфтордиарилов из перфторарилцинкорганических соединений и перфтораренов // Всероссийская научная конференция ″Современные проблемы органической химии″, Новосибирск (Россия). 2007.Тезисы докладов.С-58. 21. Brooke G.M. The preparation and properties of polyfluoro aromatic and heteroaromatic compounds // J. Fluor. Chem. 1997, 86, 1. |
Fluorine Notes, 2009, 66, 5-6