Fluorine Notes, 2003, 29, 1-2
Perfluoroalkanesulfonic acids derivatives:
synthesis and application
G.G. Furin
Novosibirsky Organic Chemistry Institute of N.N. Vorozhtsov, Siberian department of Russian Academy of sciences,
Ak. Lavrentiev avenue, 9, Novosibirsk, Russia, 630090
Fax: +7 3832 344752
E-mail : furin@nioch.nsc.ru
Abstracts
Information of the last 10 years regarding methods of synthesis and properties of perfluoroalkanesulfonic acids and some of its derivatives is listed and analyzed here. The opportunities of using the perfluoroalkane halides and syntons on the basis of perfluoroalkylsilicon derivatives for these purposes are uncovered. The main attention is paid to practical aspects of perfluoroalkanesulfonic acids salts and bis(perfluoroalkylsulfonyl)imides using as catalysts of different processes, electrolytes, ionic liquids and N-F bond containing compounds as mild fluorinating reagents. The ways of using of perfluoroalkanesulfonic acids derivatives in organic synthesis are discussed.
Table of contents
1.Introduction. The role of organic compounds in the synthesis of semi-products and the creation of new materials on their basis.
2. Perfluoroalkanesulfonic acids synthesis
2.1. Electrochemical fluorination of alkyl
derivatives of hexavalent sulfur
2.2. The use of perfluoralkyl iodides in the synthesis
of perfluoroalkanesulfonic acids
2.3. The reactions of fluorocontaining С-nucleophilic
reagents with haloid derivatives of sulfur
2.4. The use of trimethyl(trifluoromethyl)silane
in the synthesis of trifluoromethylsulfonic acid and its derivatives.
3. The synthesis and characteristics of perfluoroalkanesulfonic acids derivatives
3.1. Haloanhydrides of perfluoroalkanesulfonic acids
3.2. Bis(perfluoroalkylsulfonyl)imides
and their use in fluoroorganic synthesis
3.3. Perfluoroalkanesulfonic acids salts as catalysts of several chemical processes.
3.4. The synthesis of N-F containing compounds.
4. The new applications of perfluoroalkanesulfonic acids derivatives
4.1.
Electrolytes and ionic liquids on the basis of bis(perfluoroalkylsulfonyl)imides
4.2.
Membranes on the basis of perfluoroalkanesulfonic acids
Conclusion
References
3.3. Perfluoroalkanesulfo-acid salts, the catalysts of a number of chemical processes.
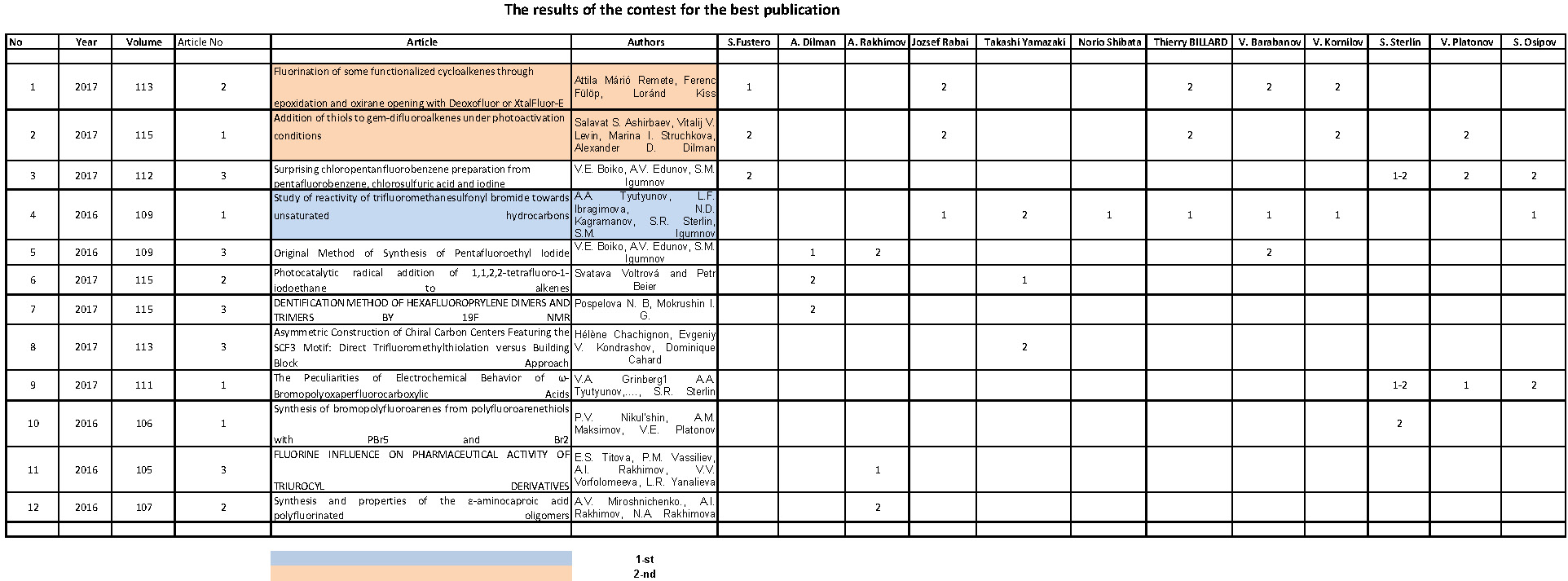
The main obtaining methods of perfluoralkanesulfo-acids and bis(perfluoroalkanesulfonyl)imides are their interaction with corresponding oxides of elements. Thus, the reaction with bismuth oxide in the media, containing the mixture of water and ethyl alcohol [110,111] or bismuth compound [112] produces corresponding bismuth derivatives. It's better to use C1 - C3 the alcohol mixable with water, the optimal ratio water/alcohol from 1:1 up to 9:1, and molar ratio of CF3SO3H/Bi2O3 is 6:1 - 6.5:1 (yield 97 %). Such salts are used as catalysts for carrying out chemical reactions of different types.
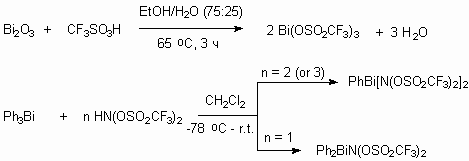
They are new ligands for lanthanide complexes creating, especially neodymium 3 [113], ytterbium and yttrium [114,115], lanthanum [116-118], europium [90] and other metals (B, Al, Ga, In, Tl, Si, Ge, Sn, Pb, As, Sb, Bi, Te) [119,120]. These complexes are excellent catalysts of Friedel-Crafts and in the last years there was found an application for them.. Eu[N(SO2C8F17)2]3 produces luminescence with quantum yield 56.8 % [90].
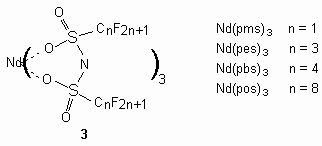
As a rule, these complexes are generated during boiling of oxides of these elements with bis(perfluoroalkanesulfonyl)imide in water for example for lanthanum:
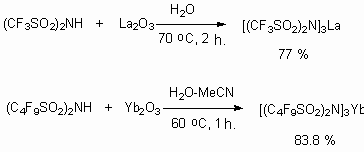
The ytterbium salts of tris(perfluoroalkanesulfonyl)methidine were obtained according to the following scheme [121:
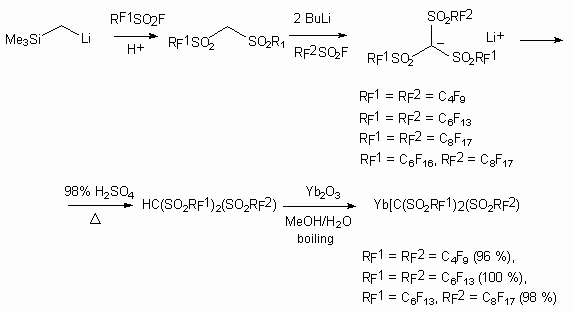
The more detailed review of data regarding application of perfluoroalkanesulfoacids is given in review [122]. We will not go beyond few new examples of their application. Thus, trifluoroalkanesulfonates of rare-earth metals are effective catalysts for example of benzyl alcohol etherification using aliphatic alcohols or thiols [123a]. In these reactions RE(OSO2CF3)3 can be easily returned into the process without loss of their catalytic activity.
It is shown [123b], that Bi(OSO2CF3)3is an effective catalyst of acetals and ketals turning into aldehydes.
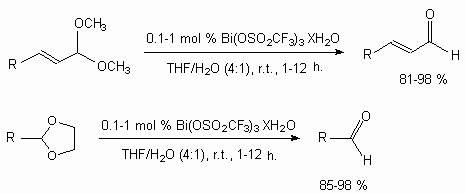
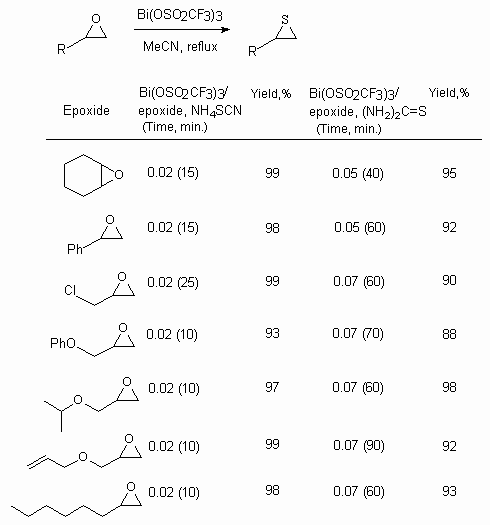
Bi(OSO2CF3)3 catalyses epoxides turning into thiirans in the presence of ammonium thiocyanate and thiourea [123c,d].
Trifluoromethanesulfonates of scandium, indium and zirconium act as effective catalysts of Friedel-Crafts alkylation of aromatic compounds by derivatives of acetylene [124].
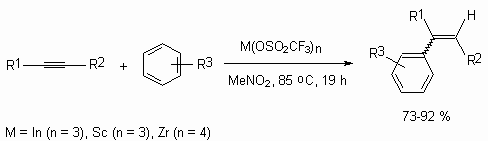
Trifluoromethanesulfonates of scandium, ytterbium and samarium appeared to be rather effective catalysts of reaction of tetrakis(allyl)germanium with carbonyl compounds, for example with benzaldehyde [125].
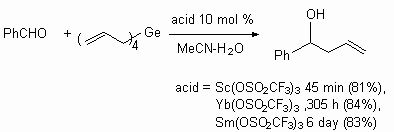
Other carbonyl compounds also enter into this reaction.
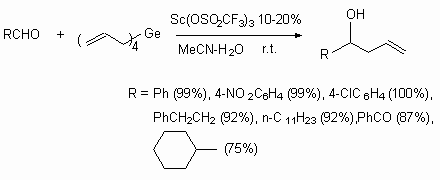
The nature of Lewis acid element is important, affecting the reaction velocity.
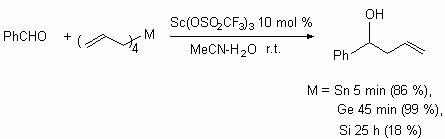
Trifluoromethanesulfonates of scandium catalyses the reaction of Diels-Alder between different acrylates and cyclopentadiene in supercritical CO2 . The reaction passes in a stereo-selective way [126].
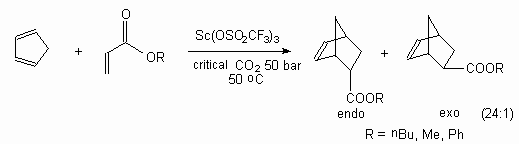
Acylation of aromatic compounds is catalysed by bis- (trifluorometansulfonyl)amides of lanthanum, samarium and ytterbium [127a,b]. At this the present catalysts appeared to be more effective compare to trifluorometansulfonates of these elements [128].
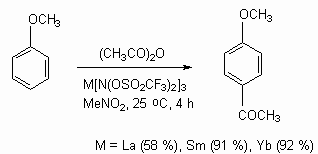
Tris(perfluorooctylsulfonyl)methidine of lanthanum and bis(perfluoroctylsulfonyl)amide of lanthanum are not only effective catalysts of alcohols and aromatic compounds acylation, but they are used as Friedel-Crafts catalysts of Diels-Alder reactions.
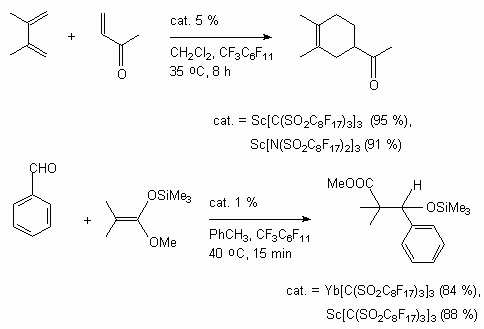
Instead of organic dissolvents supercritical carbon dioxide was used for these processes [130].
Diels-Alder reaction of different dicarbonyl compounds and 1, 2 - diens passes with high yield in mild conditions when using ytterbium trifluorometansulfonate as catalyst [131].
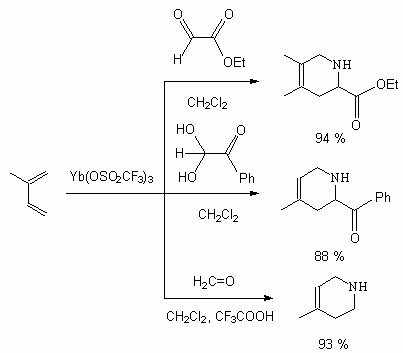
N-Derivative of bis-(trifluorometansulfonyl)imide is an effective fluorinating agent for aromatic compounds [132]. The reviews regarding synthesis of compounds, containing N-F bonds, and their use in organic synthesis you can find in [133,134].
During fluorination of N-alkylsulfonamides in the CFCl3 -CHCl3 system using fluorine [135] , CH3OF[136] or CF3OF[137,138] the corresponding N-fluoroderivatives are obtained.
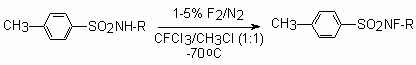
| R | Me | But | neopentyl- | exo-2-nonbornyl | endo-2-nonbornyl | cyclohexyl- |
| Yield, % | 59 | 14 | 57 | 47 | 71 | 11 |
The fluorination of sodium salt of N-tret-butyl-p-toluenesulfonamide by cesium fluoro-oxy-sulfate in acetonitrile at 0-5oC give the N-fluoro-N-tret-butyl-p-toluenesulfonamide with high yield (69%) [139]. The fluorination of this substrate using elemental fluorine produces product only with the 14% yield [135].
It is better to fluorinate sulfonamide derivatives in the form of corresponding salts. Thus, the fluorination of sodium salt of perfluoro N-(4-pyridyl)methanesulfoamide using elemental fluorine results in formation of N-fluoro-N-(4-pyridyl)methanesulfoamide [91,140].
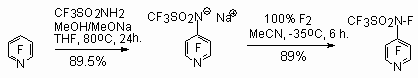
The derivatives of sulfonamides having perfluoroquinolyl-, perfluoro-iso-quinolyl- substituents [137] are put into reaction.
The controlled fluorination of aromatic compounds using N-fluorolactams and N-fluorosulfonamides can be used for obtaining of a number of aromatic fluoroderivatives.
The fluoridation of organic compounds using N-fluorosulfonamides is described by many examples [136,140]. Thus, sulfonamides of 9 type fluorinate salts of malonic acid derivatives and aromatic compounds [135,140-146].
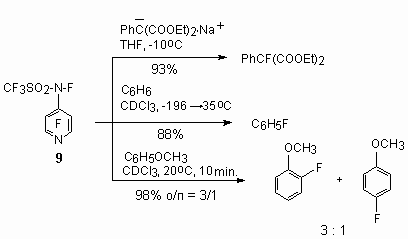
Obtaining methods of N-fluorodialkyl(aryl)sulfonimides are based on fluorination [F2/Ar (1-3%) or XeF2] of corresponding dialkyl(aryl)sulfonimides. Base products are obtained with the yield up to 70% [137].
N-fluorosulfonimides (CF3SO2)mNFRn(SO2R')p (where m=1,2; n,p= 0,1; R' - perfluoroalkyl, -cycloalkyl, -aryl) and CF3SO2NF(SO2)aR (a=0,1) are obtained using fluorination of corresponding imides by elemental fluorine at -20oC [140-146]. Thus at F2 influence on (CF3SO2)2NH at -20oC (CF3SO2)2NF is formed with the yield 94,6% [140]. The synthesis of N-fluorosulfonimides using method is described in many works [147-149].
In the article [140]it is offered to use not amides of acids, but their lithium derivatives. Authors used diluted elemental fluorine in non-polar solvents ( CHCl3, CFCl3, HF, fluorocarbon, MeCN) at 80-135oC in presence of solid phase (NaF, CaF2) [140].
N-fluorodiphenylsulfonimide was obtained during fluorination of diphenylsulfonimide by fluorine (diluted nitrogen) in presence of sodium fluoride in acetonitrile at -40oC [150].
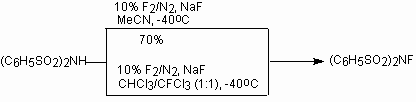
N-fluorodiphenylsulfonimide is a white crystal substance, dissoluble in plenty of organic solvents (ether, THF, CH2Cl2, MeCN, C6H5CH3), convenient in handling, non hydroscopic and stable for storage. N-fluorodimethylsulfonimide, another N-fluorodialkylsulfonimides [(CH3(CH2)nSO2)2NF, n = 1-3] and N-fluoro-[1,3,2]-dithiazine-1,1,3,3-tetraoxide was produced similarly[151]. Water or water mixture with organic solvent can be used as media. Best results are achieved with sodium salts of corresponding sulfonamides [152]. Thus, N-fluorodimethylsulfonimide is obtained at action of elemental fluorine (F2/N2 = 1:9) in acetonitrile in presence of sodium fluoride at -40oC with the yield 90% [153].
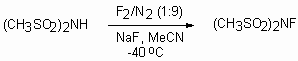
At fluorination of sodium saccharin salt by caesium fluoro-oxy-sulfate in acetonitrile at 0-5oC N-fluorosaccharin is obtained with the yield of 69%, while fluorine influence on this compound in methyl alcohol at -60 - -70oC results in uncovering of heterocycle and formation of o-fluorosulfonylmethylbenzoate (yield 28%) [154].
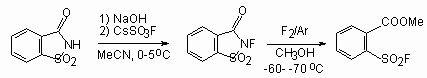
Fluorine-containing analogue of saccharine 10 is obtained by influence of diluted fluorine on sodium salt of benz-1,2,3-oxathiazine-4(3H)-one-2.2-dioxide in acetonitrile at low temperature in presence of sodium fluoride [155,156]. This reagent 10 is a crystal substance of sufficient stability and has a high capability for fluorination. Mainly it is used for fluorination of different salts, steroids and aromatic compounds.
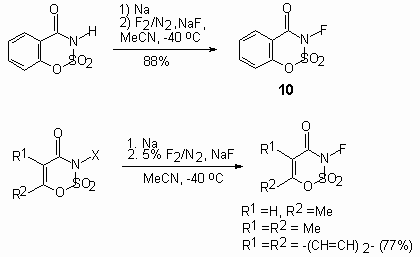
The most active agent among N-fluorosulfonimides is N-fluoro-bis-(trifluoromethanesulfonyl)amide, obtained according the above scheme with total yield 76% . This compound has a boiling point 90-91oC and is thermally stable, it can be kept in glass and is rather effective agent for fluorination with wide field of application [147-149].
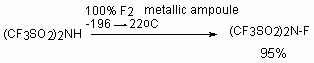
Marked with isotope 18F N-fluoro-bis-(trifluoromethanesulfonyl)amide is obtained according to the same scheme (radiochemical yield 45%) [157].
Stable white crystal powder of N-fluoro-o-benzenedisulfonimide 11 [158-160] is obtained by action of fluorine diluted by nitrogen upon î-o-benzenedisulfonimide at low temperatures.
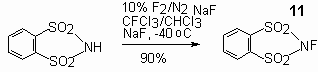
N-F-containing compounds reveal high activity as fluorinating agents [161].
to be continued
Fluorine Notes, 2003, 29, 1-2