Fluorine Notes, 2003, 29, 3-4
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Reaction N |
The rate constants Arrhenius meters of reaction |
Rate constants dimention |
|
|
lg A |
E act, kJ/mol | ||
| 1 | 12,4 | 214,0 |
sec-1 |
| 2 | 6,8 | 24,0 |
l/mol*sec. |
| 3 | 13,0 | 219,0 |
sec-1 |
| 4 | 8,4 | 32,0 | l/mol*sec. |
| 5 | 9,0 | 4,2 | l/mol*sec. |
| 6 | 16,7 | 295,0 | sec-1 |
| 7 | 8,5 | 4,2 | l/mol*sec. |
| 8 | 13,0 | 274,0 | sec-1 |
| 9 | 7,4 | 4,2 | l/mol*sec. |
| 10 | 13,0 | 285,0 |
sec-1 |
| 11 | 14,2 | 135,0 |
sec-1 |
| 12 | 14,3 | 6,0 |
sec-1 |
| 13 | 9,0 | 8,0 | l/mol*sec. |
| 14 | 6,5 | 40,0 | l/mol*sec. |
| 15 | 6,5 | 36,0 | l/mol*sec. |
| 16 | 7,7 | 24,0 | l/mol*sec. |
| 17 | 6,5 | 39,0 | l/mol*sec. |
| 18 | 7,7 | 96,0 | l/mol*sec. |
| 19 | 10,5 | 193,0 |
sec-1 |
| 20 | 7,5 | 46,0 | l/mol*sec. |
| 21 | 7,5 | 46,0 | l/mol*sec. |
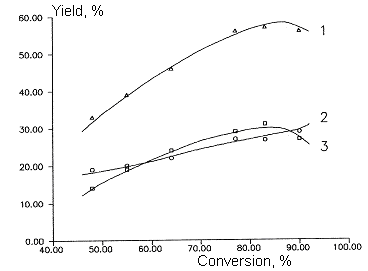
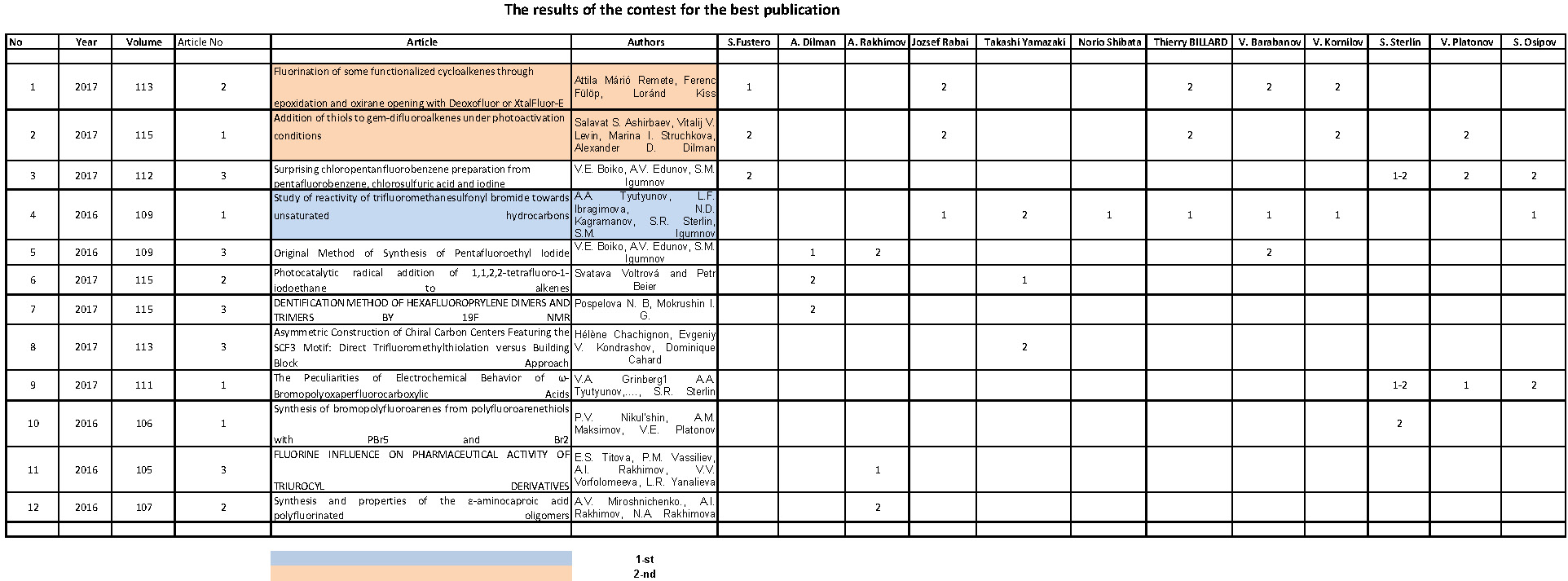
Fig 1.
Dependence of main products yields of chlorodifluoromethane and
dichlorofluoromethane copyrolysis from initial product conversion. T=
1008 K
Molar ratio of CF2ClH/ CFCl2H - 3:1
Molar ratio of aqueous vapor/sum of freons - 5:1
1- sum C2F3Cl + C2F4
, 2- C2F4, 3- C2F3Cl
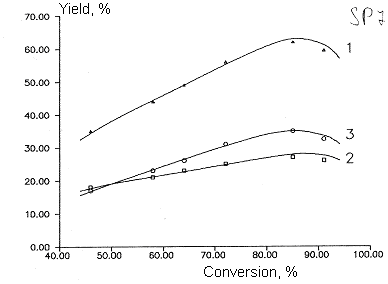
Fig 2. Dependence of main products yields of
chlorodifluoromethane and dichlorofluoromethane copyrolysis from initial
product conversion. T=1053 K
Molar ratio of CF2ClH/ CFCl2H - 2:1
Molar ratio of aqueous vapor/sum of freons - 5:1
1- sum C2F3Cl + C2F4
, 2- C2F4, 3- C2F3Cl
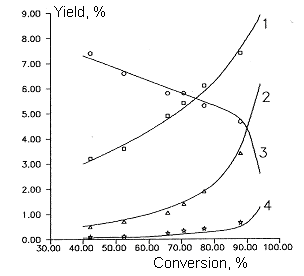
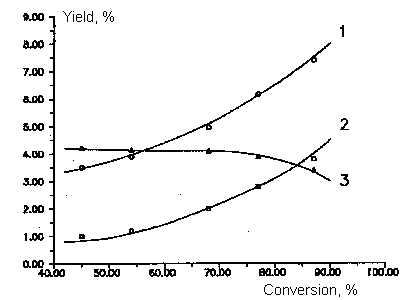
Fig 3.
Dependence of by- products yields of chlorodifluoromethane and
dichlorofluoromethane copyrolysis from initial product conversion.
T=1053 K
Molar ratio of CF2ClH/ ÑFCl2H - 3:1
Molar ratio of aqueous vapor/sum of freons - 5:1
1- CO , 2- C3F6, 3- C2F2Cl2
,4- C2F4ClH
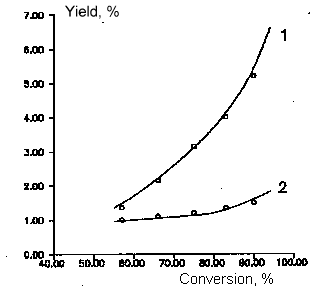
Fig
4. Dependence of by- products yields of chlorodifluoromethane and
dichlorofluoromethane copyrolysis from initial product conversion.
T=1008 K
Molar ratio of CF2ClH/ CFCl2H - 3:1
Molar ratio of aqueous vapor/sum of freons - 5:1
1- CO , 2- C3F6, 3- C2F2Cl2
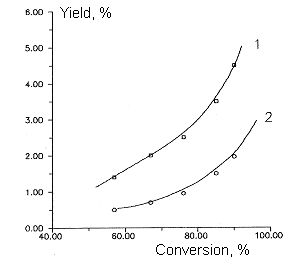
Fig
5. Dependence of by- products yields of chlorodifluoromethane and
dichlorofluoromethane copyrolysis from initial product conversion.
T=1053 K
Molar ratio of CF2ClH/ CFCl2H - 2:1
Molar ratio of aqueous vapor/sum of freons - 5:1
1- C3F4Cl2 , 2- C3F5Cl
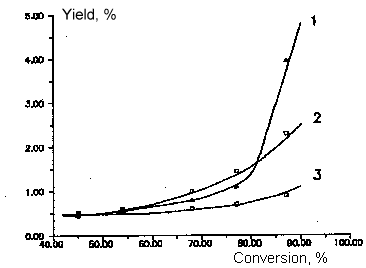
Fig
6. Dependence of by- products yields of chlorodifluoromethane and
dichlorofluoromethane copyrolysis from initial product conversion.
T=1008 K
Molar ratio of aqueous vapor/sum of freons - 5:1
Fig
7. Dependence of by- products yields of chlorodifluoromethane and
dichlorofluoromethane copyrolysis from initial product conversion.
T=1023 K
Molar ratio of CF2ClH/ CFCl2H - 3:1
Molar ratio of aqueous vapor/sum of freons - 5:1
1- C3F6 , 2- C3F5Cl,
3- C2F4ClH
Fig
8. Dependence of by- products yields of chlorodifluoromethane and
dichlorofluoromethane copyrolysis from initial product conversion.
T=1023 K
Molar ratio of CF2ClH/ CFCl2H - 2:1
Molar ratio of aqueous vapor/sum of freons - 5:1. Conversion 87%
1-C3F6 , 2-
CO, 3- C3F5Cl ,4- C3F4Cl2,5- C2F4ClH
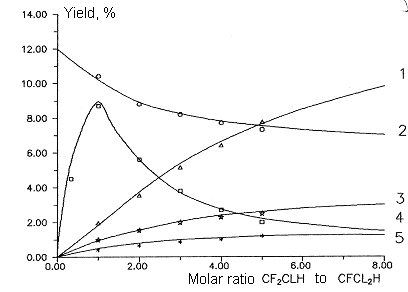
Fig
9. Dependence of main products yields of chlorodifluoromethane and
dichlorofluoromethane copyrolysis from molar ratio chlorodifluoromethane
to sum of chlorodifluoromethane and dichlorofluoromethane. T= 1023 K
Conversion 87%.
Molar ratio of aqueous vapor/sum of freons - 5:1
1- sum C2F3Cl,C2F4
and C2F2Cl2 , 2-
sum C2F3Cl and C2F4 , 3-
C2F4 , 4 - C2F3Cl , 5- C2F2Cl2
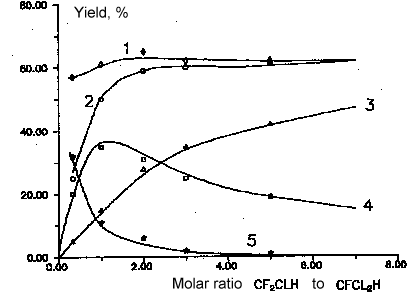
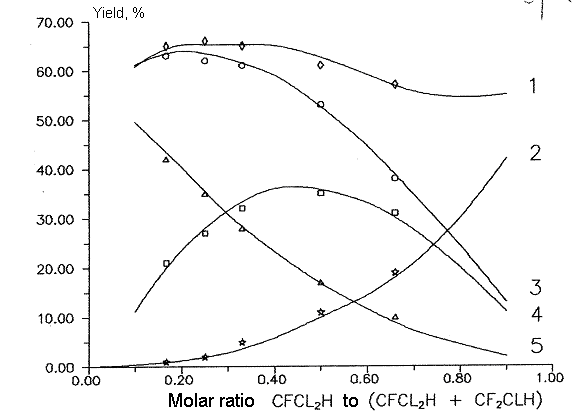
Fig 10. Dependence of main products yields of
chlorodifluoromethane and dichlorofluoromethane copyrolysis from molar
ratio chlorodifluoromethane to dichlorofluoromethane. T= 1023 K
Conversion 87%.
Molar ratio of aqueous vapor/sum of freons - 5:1
1- sum C2F3Cl,C2F4
and C2F2Cl2 , 2-
sum C2F3Cl and C2F4 , 3-
C2F4 , 4 - C2F3Cl ,
5- C2F2Cl2
Some general conclusion can be drawn based on the calculation results:
- Yield of chlorotrifluoroethylene is 95% under conversion of 85-87%
- Maximum yield of basic monomer sum is under conversion of 83-87% as well
- The molar ratio if chlorodifluoromethane to dichlorofluoromethane have main influence on yield of chlorotrifluoroethylene and other products
- Maximum selectivity of chlorotrifluoroethylene is 42,5 %, it reaches under this ratio is about 1,2 (xo=1,2)
- It is possible to produce chlorotrifluoroethylene, tetrafluoroethylene and 1,2-dichlorodifluoroethylene over a wide range of concentration in pyrolized product
The experiments on joint thermal decomposition in the presence of water vapor were carried out in a continuous reactor made of nickel-chromium corrosion-proof alloy , 4mm diameter, 500mm length of the working zone. To avoid cracking of protective oxide film on the reactor surface the heating of the latter was carried out linearly at a rate below 100K/hour. The pyrolysis was held within a temperature range of 973-1173K at a mole ratio of vapor/ freon 10:1-1.5:1.
The analysis of gas mixtures after purification from acid admixtures was made on a "Tsvet-100M" chromatograph with using a flame ionization detector and a heat conductivity detector (helium as a gas carrier, 3m column length, 2mm column diameter, 293-323K column temperature, tricrezylphosphate ( 15% on silochrome-80)).
The identification of the pyrolysis products was made by a spectrometry method on a HP-5995 instrument ( 70eV energy of ionizing electrons, 553K separator temperature, 423 temperature of the ion source)
Literature
1. Nikolaev A.A., Barabanov V.G. Study of the process of joint thermal decomposition of chlorodifluoromethane and dichlorofluoromethane. // Fluorine notes - 2002-Vol.4.
2. Gill P.E., Murroy W., Wright M.H. Practical Optimization. - London, New-York,1981.-371 p.
3. A sistematized collection of ODE Solvers: Report UCRL / Lawrence Liwermore Laboratory. N -88007,-1982.-10 p.
Fluorine Notes, 2003, 29, 3-4